A growing body of evidence supports a connection between risk factors for cardiovascular disease and cancers (1). Myocardial infarction (MI) and even cardiac remodeling have been reported to be linked to cancer progression, though more than one mechanism may be responsible (2, 3). Thus, further research into the relationship between molecular cardiology and cancer biology is essential for improved clinical management of patients at risk for heart disease and malignancy.
A new study by Yuan et al. adds an important dimension to our understanding of MI-induced heart failure (HF) and cancer by uncovering an exosome-mediated communication pathway that leads to decreased tumor cell susceptibility to ferroptosis (4). Ferroptosis, a non-apoptotic form of regulated cell death, holds promise as a potential therapeutic target to be leveraged against drug-resistant tumors. Using mouse models and patient samples, the researchers utilized several experimental strategies to identify exosome-mediated communication between cardiomyocytes in their HF mouse model and tumor cells. Examination of the HF exosomes cargo revealed enrichment of the miR-22-3p miRNA that targets ACSL4, a key pro-ferroptotic gene. These HF-associated exosomes were able to curb tumor cell sensitivity to ferroptosis inducers in both lung carcinoma and osteosarcoma cell lines in vitro, as well as in a xenograft tumorigenesis model in vivo. Suppression of cardiomyocyte-specific miR-22-3p increased ferroptosis susceptibility of the tumor cells. Thus, Yuan and colleagues have elucidated a mechanistic basis for tumor cell resistance to ferroptosis in the context of HF, and revealed another aspect of the association between heart disease and cancer progression.
GeneTex continues to expand its catalog of antibodies to support both cancer biology and exosome research, including the CD81 antibody (GTX101766) and TSG101 antibody [4A10] (GTX70255) cited in the Yuan et al. study. For more information, please see the highlighted products below and visit www.genetex.com.
Highlighted Products
|
|
|
|
|
![Glutathione Reductase antibody [N2C2], Internal (GTX114199) Glutathione Reductase antibody [N2C2], Internal (GTX114199)](/upload/media/MarketingMaterial/Newsletter/2023/CD63-TSG101/255x255_05.webp) |
Glutathione Reductase antibody [N2C2], Internal (GTX114199)
|
   |
|
|
|
|
References:
- Cardiovasc Res. 2019 Apr 15;115(5):844-853. doi: 10.1093/cvr/cvz035.
- Cells. 2022 Mar 25;11(7):1108. doi: 10.3390/cells11071108.
- Nat Med. 2020 Sep;26(9):1452-1458. doi: 10.1038/s41591-020-0964-7. Epub 2020 Jul 13.
- Signal Transduct Target Ther. 2023 Mar 27;8(1):121. doi: 10.1038/s41392-023-01336-4.
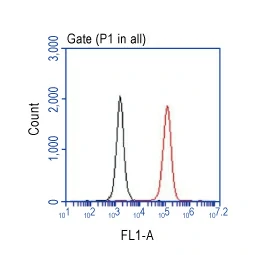
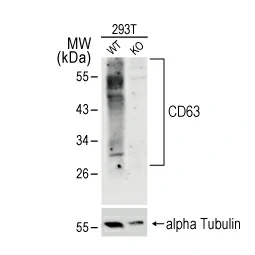
![TSG101 antibody [4A10] (GTX70255) TSG101 antibody [4A10] (GTX70255)](/upload/media/MarketingMaterial/Newsletter/2023/CD63-TSG101/255x255_02.webp)
![FACL4 antibody [HL229] (GTX635616) FACL4 antibody [HL229] (GTX635616)](/upload/media/MarketingMaterial/Newsletter/2023/CD63-TSG101/255x255_04.webp)
![Glutathione Reductase antibody [N2C2], Internal (GTX114199) Glutathione Reductase antibody [N2C2], Internal (GTX114199)](/upload/media/MarketingMaterial/Newsletter/2023/CD63-TSG101/255x255_05.webp)
![NRF2 antibody [HL1021] (GTX635826) NRF2 antibody [HL1021] (GTX635826)](/upload/media/MarketingMaterial/Newsletter/2023/CD63-TSG101/255x255_06.webp)